
BACKGROUND
Almost all of the stainless steels in use have 16% chromium or more and have nickel or other additions to make them austenitic and hence formable, tough and readily weldable. However, the formal definition of a stainless steel is that it is an iron- and carbon-based alloy with more than 10.5% chromium. Historically, the corrosion mitigation industry regarded alloys with more than 12% chromium as stainless steels mainly because those alloys did not corrode in mild environments. Because of the perceived problem of high initial price when using stainless steels, alloys that are ‘barely’ stainless (and with low nickel to boot) are more competitive with painted or galvanised carbon steel than higher alloys.
HOW WERE THESE GRADES DEVELOPED?
More than 30 years ago, developments from the 409 grade (used for car exhausts) led to a weldable ferritic that was tough to sub-zero temperatures. Two versions were developed: a stabilised grade for corrosive environments and an unstabilised grade that matched international standards. One issue was that the titanium used for stabilisation was hard on the refractories and caused the surface finish of flat product to be less appealing. However, when end users moved to unstabilised versions, corrosion problems arose in some applications. Research lead to further alloy development and proprietary grades with outstanding resistance to weld sensitisation.
WHAT IS DIFFERENT ABOUT THESE MATERIALS?
- They are ferritic (and attracted to a magnet), and can be bent, formed, cut and electric process welded like carbon steels.
- The balance of their metallurgy limits grain growth when heated. So, unlike ferritics used for cladding, thick sections can be welded without excessive grain growth and embrittlement.
- After welding, they have a duplex ferritic-martensitic microstructure that does not usually require heat treatment.
- As ferritics, their thermal expansion is low (actually less than carbon steel) which reduces distortion risk during welding or furnace operations.
- They have good scaling resistance in air to ~600˚C and reasonable strength at that temperature compared with more expensive austenitics with a scaling limit of ~800˚C in air.
- Like duplex alloys, they do not suffer from chloride stress corrosion cracking.
- They provide excellent and economic resistance in corrosive wear applications compared to hardenable carbon steels, surface-treated materials of highers alloys.
However, there are a few cautions:
- Low chromium, low nitrogen and no molybdenum means they have low corrosion resistance (PRE~11). They will pit in marine environments and in less severe conditions they cannot be used if aesthetic appearance is critical. Painting is a useful option in aggressive environments.
- Neither cold work nor heat treatment will increase their strength, although they are slightly stronger than 300 series stainless steels. Because they do not cold work, they should be less susceptible to galling then austenitic stainless steels.
- While it is nothing to do with the material, supply is mostly limited to sheet or plate, i.e. bar, hot-formed sections, hollow sections and wire and generally unavailable.
WHAT ARE THE ALLOYS?
There is a plethora of proprietary and standardised grades with between 10.5% and 12% chromium. The Ferritic Solution booklet available  from the ISSF [www.euro-inox.org/pdf/map/The_ferritic_solution_EN.pdf] lists about a dozen. In Australia, the major proprietary grades are 3Cr12 and 5Cr12 where the ‘3’ and ‘5’ are labels, not compositions, and may include additional letters for other grades in the family. However, these labels cover three different material design decisions – and only those in (A) below are standardised:
from the ISSF [www.euro-inox.org/pdf/map/The_ferritic_solution_EN.pdf] lists about a dozen. In Australia, the major proprietary grades are 3Cr12 and 5Cr12 where the ‘3’ and ‘5’ are labels, not compositions, and may include additional letters for other grades in the family. However, these labels cover three different material design decisions – and only those in (A) below are standardised:
A. Low chromium, no molybdenum and low nickel, carbon and nitrogen. There are covered by S40977/1.4003 in ASTM A240/EN10088.2
respectively or S41003 in ASTM A240.
B. As above, but with stabilising titanium or titanium plus niobium. There are several rules for titanium content but 4 (C+N) with a limit of 0.6 is used. The Ti/Nb will lock up C and N and reduce the risk of sensitisation, i.e. it limits corrosion associated with welds.
C. As above, but with lower carbon and nitrogen limits and specific controls on ferrite and austenite stabilising elements. This gives immunity to sensitisation in corrosive environments where there is a risk of fatigue.
REPLACEMENT OF GALVANISED OR COATED CARBON STEEL BY Cr12
The cost of steel that has been galvanised is currently up to 30% less than the cost of a 12Cr utility stainless steel when transport, pickling and other costs are included. When added to the cost of better trained (and hence more expensive) staff required for fabricating stainless steel, it is apparent that on a prime cost basis, even this basic stainless steel will not be cost competitive. However, on a LCC basis, the 12Cr grades have a significant advantage primarily because of durability.
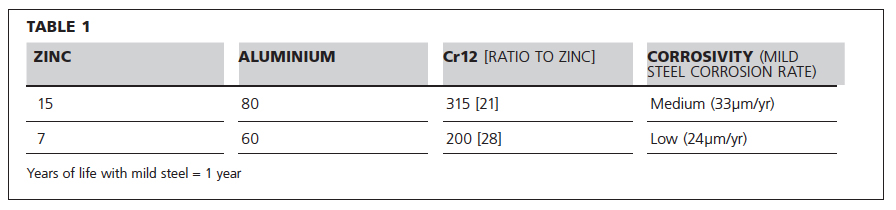
Table 1 shows the relative lifetime of zinc (as a proxy for galvanising) and aluminium vs a 12Cr stainless steel in a medium and low corrosivity environment where the atmospheric corrosion rates for carbon steel are listed averaged over a 20-year exposure. It is clear that the life cycle cost of the 12Cr stainless steel is much better than either of the alternatives listed.
 WELDING OF Cr12 STAINLESS STEELS
WELDING OF Cr12 STAINLESS STEELS
AS/NZS 1554.6 deals with welding of structural stainless steels and compacts all three branches of the 12Cr grades under ‘1.4003’ for selection of consumables. The recommendation is to use a 309L consumable although 18-8Mn (Note 8) is also prequalified. Heat input should be between 0.5 and 1.5kJ/mm and the interpass temperature should not exceed 150˚C.
As with all stainless steels, contamination by carbon steels must be avoided and any heat tint should be removed prior to exposure to corrosive service. While owners using Cr12 alloys for corrosive abrasion service regard the in-service removal of heat-tint surface layers as sufficient, this is only true if sufficient material is removed to expose the virgin stainless steel before the first rest period with corrodents on the surface could promote pitting.
APPLICATIONS FOR 12Cr STAINLESS STEELS
Applications include piggeries, rail cars, road transport, sugar and mineral industry (especially with corrosive wear), effluent tanks, under pans for conveyors, ducting (including furnaces), BBQ plate, electrical meter boxes, floor plates, gravel screens, railway overhead support towers, etc.
ACKNOWLEDGEMENTS
This paper has been prepared with support from ASSDA colleagues and especially Acerinox, Atlas Steels and Sandvik. Their assistance is gratefully acknowledged.
This technical article is featured in Australian Stainless magazine, issue 52.