
Raw material price fluctuations and increasing demand for stainless steels have driven demand for lower cost alloys as alternatives to the traditional “300” series steels. This has been met through a range of existing and new, innovative steels with different properties, performance and availability broadening the range of alloys that might be found in the market. But as with the traditional stainless steels you can’t tell what they are by looking at them.
This article describes most of the range of test methods available for grade confirmation. The method used depends on the budget, size of job and the potential consequences of having the wrong alloy.
Why test?
Contract documents may require formal test certificates. Usually these are issued by the mill and unless there is reason to doubt them this is sufficient. However, sometimes a positive material identification (PMI) is required for safety critical items such as LPG valves. Legal cases also tend to be very demanding about precise documentation. Some products may also be lacking in documentation and traceability.
Unexpected poor performance often prompts calls for material testing. Such testing removes one variable in things that might have gone wrong but the cause is more frequently inadequate surface finish or errors in design or fabrication.
Finally, reverse engineering of an existing product from a competitor or overseas supplier often requires detailed materials’ information.
What level of testing is required?
General or intermediate level guidance could cover differentiation between carbon and stainless steel or between 304 and 316 or between 300 series and 200 series or ferritic grades.
Full laboratory chemical analysis will be needed for some cases (such as determining low carbon grades) or when it has become a legal rather than a technical issue.
Full mechanical and metallurgical analyses may also be required if strength or hardness are essential design elements. If the material has undergone subsequent surface modification then the required investigation could be extensive – and expensive.
Simple physical tests
Appearance is not a reliable indicator of the grade of stainless steel as the differences are determined more by surface treatments than alloy composition. Even the differences between mirror polished surfaces are fairly subtle. The table below shows slight differences in density of some stainless steel alloys but density determination is not a convenient method.
|
Alloy |
Density (g/cc) |
|
430, 3Cr12/5Cr12 |
7.7 |
|
2205 |
7.8 |
|
304, 310 |
7.9 |
|
316 |
7.98 |
A widely accepted test is a magnet. Duplex, super duplex, martensitic and ferritic stainless steels are strongly attracted to a magnet while annealed austenitic stainless steels are not. However, cold worked austenitic stainless steels are weakly attracted to a magnet so cold formed ends to a vessel, cold rolled bolts and bent corners will be affected by a magnet. This applies to both the conventional chromium-nickel 300 grades and the chromium-manganese 200 series austenitic grades.
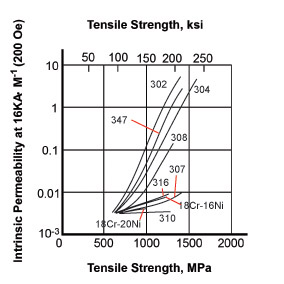
The strength of the effect that a magnet has on a material can be related to the relative permeability and the graph shows the different effect of the same level of cold work (bending) on various austenitic alloys. The grades with higher nickel or austenitising elements (310 or 316) show much lower magnetic properties. In comparison, mild steel has a relative permeability somewhere between a few hundred and 2000. Relative permeability of duplex and ferritic alloys is in the hundreds. Precipitation hardening alloys are magnetic but the degree depends on whether the alloy is martensitic or semi-austenitic.
Chemical tests
The proprietary kits are designed to test for a specific element and have a limited shelf life. If you have a project requiring multiple tests then they are very useful. However, if you only require a couple of tests a year, then it may be cheaper and more thorough to run a full laboratory test.
Molybdenum
The most common test uses a single drop of solution to distinguish between low and high molybdenum content. The “Moly Drop” test will distinguish between 304/304L and 316/316L but the test will also give a positive result with 317/317L, 904L, the 6% Mo grades, 444, 2205 and the super duplex grades. The test requires a clean, dry, grease free surface and it sometimes helps to lightly abrade the surface.
The yellow drop (as shown) will darken after a few minutes but the reaction speed is slower if the surface is cold. It is a comparative test and scrap additions during production may give enough molybdenum to give a slight colouration.
The test is therefore most reliable if a known 304 and 316 are tested with the unknown. If the sample is to be used in service, then the chemicals should be washed away immediately after the test.
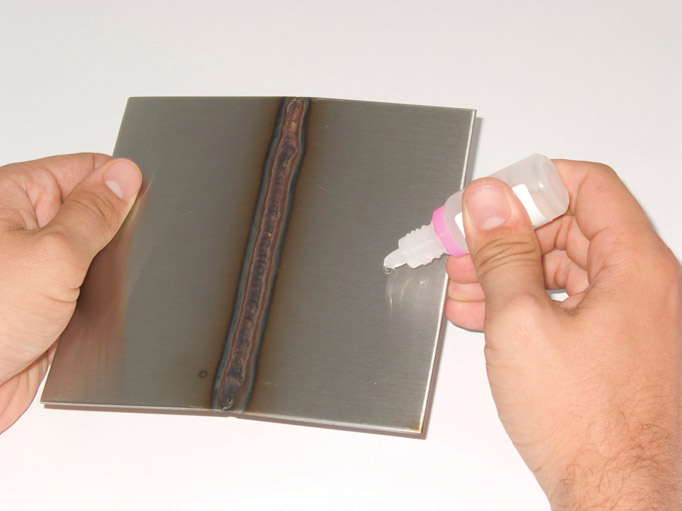
There is another chemical test using ethyl xanthogenate to form a red or pink complex when molybdenum ions are dissolved in solution. The molybdenum is dissolved from the surface either by using a hydrochloric or sulphuric acid. The strength of the colour depends on the level on molybdenum in the alloy.
Manganese
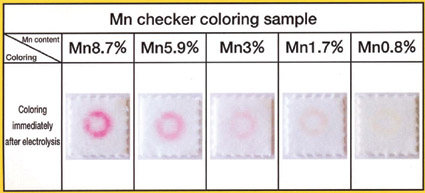
The increasing use of high manganese stainless steels has led to several manganese test kits operating on the same principal as the electrochemical test for molybdenum. The semi-quantitative results of a kit test for manganese are shown in the photographs below.
Apart from the recent low nickel, high manganese stainless grades, there have been specialist 200 series grades used in generators, higher strength (pre duplex) marine alloys and for anti-galling applications.
 Sulphur
Sulphur
A practical and rapid test for a high sulphur (free machining) stainless steel (303 and 430F are the most common) is to prepare sulphur prints using photographic paper soaked in 3% sulphuric acid for several minutes. The treated paper is pressed onto a cleaned surface for about 5 seconds. High sulphur levels are shown by a brown colour. Once again, this is a comparative test so low and high sulphur samples should be compared to the test piece.
Instrumental techniques
There are two basic techniques each with two variants. The automated instruments are expensive and would normally be used for large projects, or by scrap metal merchants, manufacturers or specialist NDT contractors.
Spark spectroscopy requires a flat surface preferably about 20mm in diameter. An electrical spark is generated and the colour of the spark is determined by the elements present. The elemental concentration is controlled by the intensity of the specific colours. In automated instruments, the spectrum is compared to a library of data and percentage composition is calculated for each element. Calibration is required against materials with similar composition. A sparking mark is left on the surface and must be removed if appearance or fatigue resistance is important. The instrument’s accuracy tends to be lower than a laboratory instrument and exposure to air excludes measuring nitrogen.
The older “Metascopes” were also spark spectroscopes but relied on visual comparisons of line brightness so their accuracy was very operator dependant. Grinding spark identification using a hard, high speed grinding wheel is even older technology. It will cause a grinding burr and is extremely dependent on the operator skill. Spark bursts are related to the carbon content and characteristic sparks/carrier lines are related to the alloying metals. Chromium in steel produces a spark stream that is orange-red in colour. A yellow colouration caused by nickel persists all along the spark whereas the orange specks of chromium appear only near the origin of the spark stream, in close contact with the grinding wheel. Relatively narrow and short spark streams, white-yellow in colour, are produced in type 304 stainless steels.
The second broad method is X-ray fluorescence. Older instruments used one or more radioactive sources although more recent miniaturisation of X-ray tubes means that some instruments generate X-rays directly. Regardless of their source, the X-rays excite electrons from the inner shells of the elements and when outer electrons fall into the newly vacant shell, a characteristic spectrum of light – generally with a number of lines – is emitted.The instrument measures the intensity of counts in each line and compares it to an internal databank. Provided that the surface is clean and smooth and the measurement is for long enough to give good statistics (typically between 20 and 60 seconds), then the alloy can be identified. However, because of the physics of X-ray fluorescence, it cannot analyse for light elements, especially carbon or nitrogen. The units are light and easy to use as seen in the picture. One advantage for reporting is that results can be directly downloaded into a computer records system
Laboratory measurements
Atomic Absorption (AA) or Inductively Coupled Plasma Spectroscopy (ICP) techniques use laboratory instruments after a sample has been digested in (usually) a mixture of acids. This is slow and may be more expensive than a spark test but it will give a more complete and reliable result. Carbon requires a separate (LECO ignition) test and detecting silicon by either method requires aggressive chemicals to get the silicon into solution.
Which test?
- Is it 430/2205 or 304/316?
A magnet will be strongly attracted to 430 and 2205 but only weakly to deformed parts of 304 or 316. - Is it 430 or 2205?
Both are strongly magnetic but only duplex 2205 will give a positive moly drop test result. - Is it 304 or 316?
A moly drop test will give a positive result with 316. - Is it a low carbon grade?
Only a spark spectrometer can distinguish between low and standard carbon grades.
In all these cases a full laboratory analysis will answer the question and provide a full composition for about $100.
This article appeared in Australian Stainless Issue 42.
The assistance of ASSDA colleagues is gratefully acknowledged - in particular, Peter Moore from Atlas Steels.