
Humanity’s use of materials has progressed over the millennia from natural resources such as plants and stone to manufactured materials such as ceramics, metals and plastics with a corresponding increase in consumption of energy and materials – and increasing waste production. In parallel, the world’s consumers have grown exponentially from about 1 billion in 1800, to 7.6 billion in 2018 and a predicted 9.8 billion in 2050 – all demanding more infrastructure, facilities and resources to support the expectations of higher standards of living. This has led to an increasing realisation that green production, recycling, waste reduction and more efficient use of resources are essential.
The green or sustainable credentials of stainless steels largely derive from their corrosion resistance and consequent long life, without the need for more than cleaning by rain washing or routine water and detergent cleaning. A good example is the Chrysler Building in New York which was built in 1930. It has only been washed twice in 1961 and 1995 using low impact detergents and yet it still retains its bright appearance partly because of good drainable design, although the inherently smooth surface from its manufacture was also a factor.
and consequent long life, without the need for more than cleaning by rain washing or routine water and detergent cleaning. A good example is the Chrysler Building in New York which was built in 1930. It has only been washed twice in 1961 and 1995 using low impact detergents and yet it still retains its bright appearance partly because of good drainable design, although the inherently smooth surface from its manufacture was also a factor.
In comparison, the Eiffel Tower in Paris is painted every seven years using 60 tonnes of paint in a 15-month campaign with 25 painters and their consumable equipment. Closer to home, the constant repainting of the Sydney Harbour Bridge provides a similar contrast to the penetration of stainless steel into the building and construction industry without the ongoing labour required for repainting and maintenance of carbon steel structures. At a smaller scale, current practice minimises maintenance in more aggressive environments by processing the surface after fabrication as shown by the bright surface of the electropolished railings beside the Brisbane River.
It is difficult to compare any corrosion (and therefore lifetime) of stainless steel with carbon steel or zinc because of the different mode of attack, i.e. stainless steel pitting vs. the general loss of copper or zinc. However,  a South African 20-year atmospheric corrosion study of lifetimes used carbon steel as a baseline of 1 and found that zinc, copper, aluminium and 316 stainless steel had lifetimes of 25, 90, 170 and >5000 years respectively.
a South African 20-year atmospheric corrosion study of lifetimes used carbon steel as a baseline of 1 and found that zinc, copper, aluminium and 316 stainless steel had lifetimes of 25, 90, 170 and >5000 years respectively.
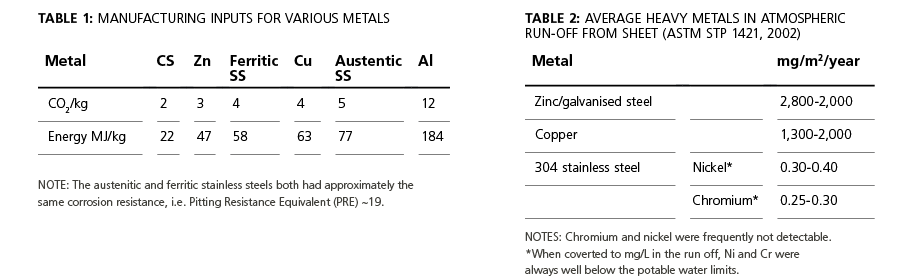
A secondary benefit of the long life of stainless steel is that the carbon dioxide emissions and the embodied energy required in manufacture are amortised over a much longer period of time. Raw CO2/kg metal and MegaJoule/kg metal data is given in Table 1 for these materials. Stainless steel is not the lowest or highest in absolute terms of carbon dioxide emissions or energy required per kilogram of stainless steel produced, but when its long life is considered, its performance on these criteria is outstanding.
Stainless steel does not use volatile organic solvents in its production or use and does not contain lead, mercury or other leachable heavy metals. Stainless steel is routinely used in pharmaceutical, food and beverage processing because of this chemical stability due to the hydrated chromium-oxide passive layer.
In a confirmation study of the stability of stainless steel with water, a 3.5-year testing program of the hot and cold water in 316 pipework of a Scottish hospital found the chromium content was less than 1% of the 0.5ppb permitted for potable water and nickel content (a trace food requirement) was less than 3% of the 0.2ppb permitted.Looking at environmental issues, Table 2 shows the results of a Scandinavian run-off study, commissioned because of concerns about heavy metals in environmentally sensitive areas. The zinc and copper values will obviously vary with time as the oxide layers form and leach. However, the passive film of stainless steel is substantially stable so that run-off can be used for potable water. A first flush discard system may also be used.
REUSE AND RECYCLING
In a well designed and executed project, stainless steel will not degrade and therefore it is probable that the process or application will become outdated while the stainless steel is still operational as a pipe or vessel or tank or other component. Such repurposing may be on the same site or elsewhere in the same industry, e.g. from milk to wine or water or fruit juices or for a radically different process. However, it is rare for repurposing to move from chemical to hygienic industries. Since stainless steel has an inherently high value, there are multiple examples of building refurbishment where the stainless steel has suffered mechanical damage or the layout must be changed. The William Penn Place (Pittsburgh) rejuvenation shown was after 50 years of use but did not require material replacement.
application will become outdated while the stainless steel is still operational as a pipe or vessel or tank or other component. Such repurposing may be on the same site or elsewhere in the same industry, e.g. from milk to wine or water or fruit juices or for a radically different process. However, it is rare for repurposing to move from chemical to hygienic industries. Since stainless steel has an inherently high value, there are multiple examples of building refurbishment where the stainless steel has suffered mechanical damage or the layout must be changed. The William Penn Place (Pittsburgh) rejuvenation shown was after 50 years of use but did not require material replacement.
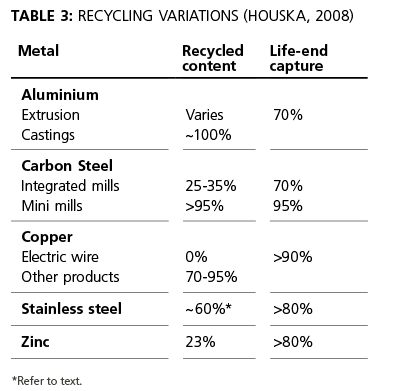
Recycling may occur as part of the life cycle, e.g. re-melting of scrap, or at end-of-life. Table 3 indicates significant variations depending on the material and its proposed use. A study of the recycling at 14 European mills covered 18 products across two ferritic, two austenitic and one duplex grade, i.e. all but the small volume of specialised, niche grades of stainless steel. For each of the 18 products, the mean recycled stainless steel content was significantly  greater than 65%. The six ferritic products were all above 90%, the nine austenitic products were between 68% and 78% while the three duplex product forms had between 69% and 76% recycled steel input.
greater than 65%. The six ferritic products were all above 90%, the nine austenitic products were between 68% and 78% while the three duplex product forms had between 69% and 76% recycled steel input.
While some mills show significantly higher percentages, a nominal 30-year life of stainless steel combined with the almost 6% compound growth of stainless steel use means there is insufficient scrap available now to substantially increase the recycled content from general use.
LIFE CYCLE COSTING AND SAVINGS FROM DURABILITY
The minimal maintenance required on stainless steel buildings and structures is a significant direct cost saving, and increased availability of equipment is also important. For example, in a waste water processing plant, a decision to replace the wetted parts of a galvanised distributor with 316 and the notionally dry parts with 304, reduced maintenance costs by 92% and increased availability from 76% to 98%.
A civil engineering example is the Progresso Pier as shown below where the original pier with carbon steel reinforcement is in ruins after 32 years exporsure. A Nickel Institute funded comparison between the 1940s construction using 304 reinforcement (right pier) and a theoretical pier constructed with carbon steel showed that the carbon steel would have contributed to a 44% greater overall life cost until 2020. It also showed that using stainless steel reinforcement had between 20% and 80% less environmental impact. This low figure was due to the predominance of the mass of concrete compared to the 240 tonne of stainless steel.
GREEN AND SUSTAINABLE
Green projects minimise energy use and one option is to reduce solar loading by installing perforated sunscreens or fixed slats in locations where insolation is high and ambient temperatures are not extreme. Design of perforated sunscreens is a sophisticated but well understood process with standard programs available. There are multiple examples that use stainless steel because it does not require more than rain or simple water washing to retain a bright appearance.
Finally, increasingly the “green” label means growing plants or other flora along stainless steel wires or supports either in public places as a visual softening or as a deciduous sun screen where stainless steel is required because of the lack of maintenance access to the supports once the vegetation is mature.
In summary, the durability of stainless steel provides substantial reductions in maintenance costs, supports a considerable recycling and reuse process, and provides control mechanisms for energy use.
This article featured in Australian Stainless magazine - Issue 63, Spring 2018