Contact between dissimilar metals occurs frequently but is often not a problem. The aluminium head on a cast iron block, the solder on a copper pipe, galvanising on a steel purlin and the steel fastener in an aluminium sheet are common examples.
Download ASSDA Technical FAQ 1 (PDF)
WHAT CAUSES GALVANIC CORROSION?
For galvanic or dissimilar or electrolytic corrosion to occur, three conditions must be met:
- The metal join must be wet with a conductive liquid
- There must be metal-to-metal contact
- The metals must have sufficiently different potentials
Wetting the join
The conductive liquid (or electrolyte) could be rainwater or water absorbed into surface deposits if the relative humidity (RH) is high enough or even simple condensation. If the deposits are sea salt, then they will start to dissolve if the RH exceeds 34% because of the magnesium chloride. The greater the conductivity the more severe the galvanic effects. Salt or industrial pollution significantly increases the conductivity of water so galvanic effects are normally more severe near the coast or in heavy industrial areas. Low conductivity, pure rainwater will only cause slight galvanic effects. One complication is that during evaporation, water films become more conductive so initially benign water may cause quite active galvanic effects as the liquid in the crevice under a bolt or clamp becomes more concentrated. Water may be excluded by design or the use of adhesive sealants or by painting the noble metal for 30 to 50 mm beyond the join to prevent charged atom (ion) transport in any thin water film. Painting the active metal (carbon steel or aluminium or zinc) can cause deep holes at coating defects.
Metal-to-metal contact
Galvanic corrosion can only occur if the dissimilar metals are in electrical contact. The contact may be direct or by an external pipe or wire or bolt. If the dissimilar metals are insulated from each other by suitable plastic strips, washers or sleeves then galvanic corrosion cannot occur. Paint is not a reliable electrical insulator especially under bolt heads or nuts or washers or near edges of sheets of metal. The paint is usually damaged on installation or by subsequent movement. Note that the chromium oxide film layer on the stainless steel is very thin and not an electrical insulator. Therefore the chromium oxide film will not prevent galvanic corrosion.
Potential differences
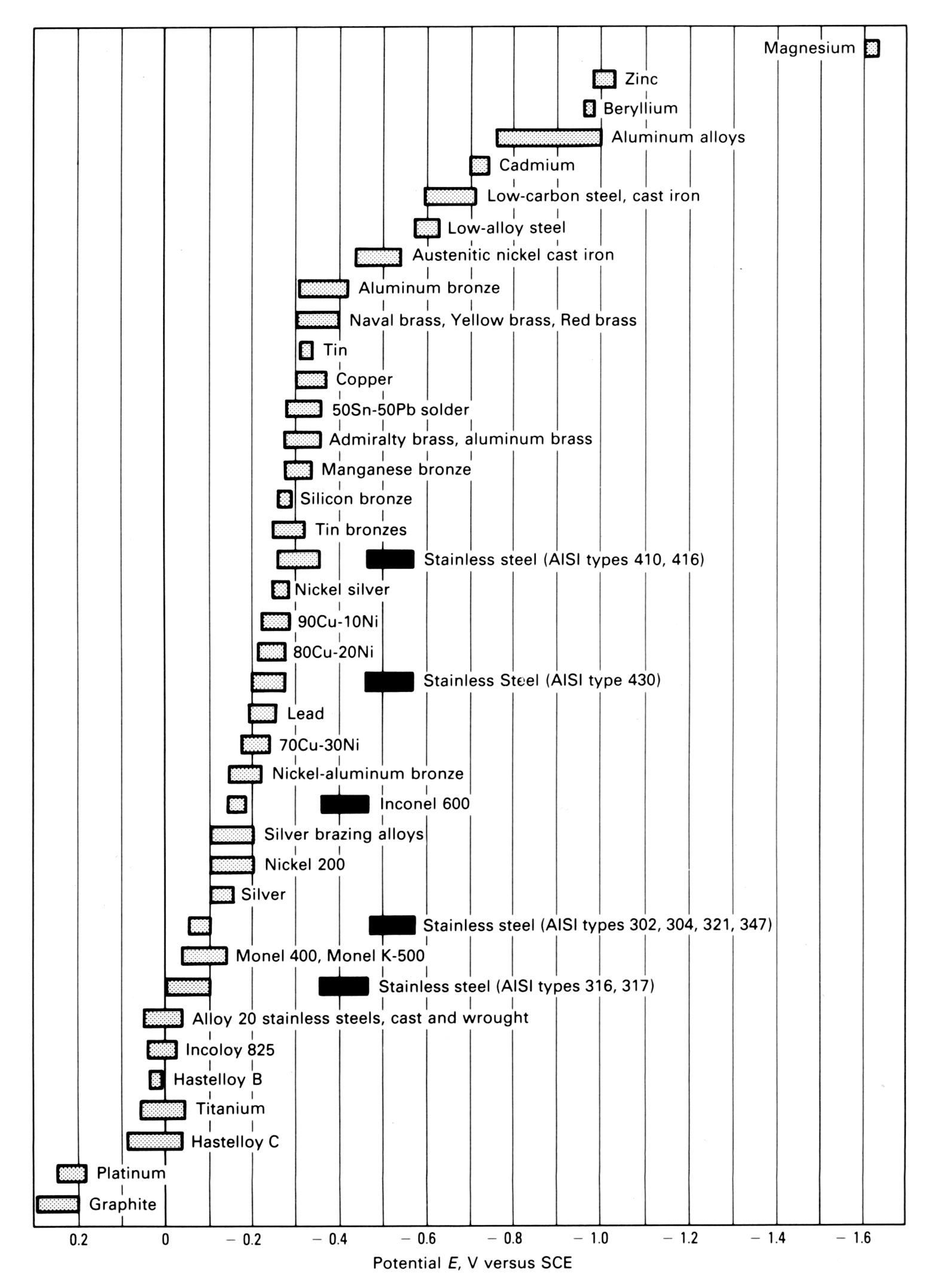
All metals dissolve to some extent when they are wetted with a conductive liquid. The degree of dissolution is greatest with active or sacrificial metals such as magnesium and zinc and they have the most negative potential. In contrast, noble or passive metals such as gold or graphite are relatively inert and have a more positive potential. Stainless steel is in the middle although it is more noble than carbon steel. The potential can be measured with a reference electrode and is used to construct a galvanic series as shown below (ASTM Standard G82).
When two metals are connected and in contact with a conducting liquid, the more active metal will corrode and protect the noble metal. Zinc is more negative than steel and so the zinc coating of galvanised steel will corrode to protect the steel at scratches or cut edges. The stainless steels, including 304 and 316, are more positive than zinc and steel, so when stainless steel is in contact with galvanised steel and is wet, the zinc will corrode first, followed by the steel, while the stainless steel will be protected by this galvanic activity and will not corrode. The rate of galvanic attack is governed by the size of the potential difference.
The graph shows that stainless steels have two ranges of potential. The usual, passive behaviour is shown by the light hatching. However, if the passive film breaks down, the stainless steel corrodes and its potential is in the dark bar range.
As a rule of thumb, if the potential difference is less than 0.1 volt, then it is unlikely that galvanic corrosion will be significant.
If all three conditions are met then galvanic corrosion is probable and the rate of corrosion will be influenced by the relative area and the current density delivered by the noble metal.
RELATIVE WETTED SURFACE AREA
If a noble metal like stainless steel has a large surface area in contact with the electrolyte while the sacrificial metal (such as aluminium) has a very small surface area in contact with the electrolyte, then the stainless steel will generate a large corrosion current which will be concentrated on a small area of sacrificial metal. The aluminium will corrode quickly, and so aluminium fasteners in stainless steel are not acceptable. However, a stainless screw in aluminium is frequently used although corrosion of the aluminium immediately around the stainless is quite possible. This is because the ratio of A wetted noble fastener in an active metal might change from a 1:50 ratio to 1:1 during drying after a rainstorm. If contaminants are significant this means that avoiding dissimilar metal pairs may be a preferred option to prevent galvanic attack.
Galvanised fasteners in stainless steel will also lose zinc more rapidly than stand alone exposures. An added disadvantage is that the corrosion product will turn from white to orange when the corrosion reaches the zinc-iron alloy near the bottom of the galvanised layer. After that, corrosion of the carbon steel fastener commences - again at a faster rate than stand alone exposures.
As a rule of thumb, if the wetted area of the corroding metal is 10 times the wetted area of the noble metal, then galvanic effects are not serious although the larger the ratio the less the effect.
AVAILABLE CURRENT DENSITY
Stainless steel has an effective passive film so the available corrosion current able to be carried by charged atoms (ions) is quite low. If the behaviour of a copper/steel and a stainless steel/steel couple is compared, the copper/steel coupling is a more significant galvanic problem despite the similar potential separation of 0.35 volts.
Examples of acceptable galvanic pairs include:
- The copper alloy potential is more active than the stainless steel and it provides cathodic protection current to limit pitting of the stainless steel shaft or crevice attack at the bearing sleeve. The depth of loss of the copper alloy is low because it has a very large area compared to the exposed shaft.
- Galvanised steel pipe hangers are used to hang stainless steel piping externally around chemical plants. The surface area ratio is bad with large area of stainless steel to small area of active zinc/steel but the rainwater is usually of quite low conductivity and 20-year service life is normal.
- In the water industries, galling between stainless steel threads and nuts has been avoided by using aluminium bronze nuts on stainless steel studs or bolts. Although aluminium bronze is more active than stainless steel, the conductivity of the water, and hence the corrosion rate, is generally quite low. The nuts will require replacement but only at times of major overhaul.
- The potential difference between passive 304 and passive 316 is small so galvanic corrosion of the 304 is not expected, even with large area ratios.
Unacceptable material pairs include a rubber seal with a carbon black loading so high (for UV resistance) that it is conductive and causes galvanic attack of a stainless screw or pin. Gaskets incorporating graphite have caused similar problems for stainless steel flanges and must not be used for seawater regardless of the stainless steel alloy. Uninsulated stainless steel fixings are not permitted for Colorbond® wall or roof sheeting as the galvanic current from the corroding Zincalume® blisters the paint.
GALVANIC SERIES