Successfully using stainless steel depends on environment, grade selected, surface finish, the expectations of the customer and the maintenance specified.
Stainless steels provide robust solutions, but in harsh or borderline environments with high expectations for durability, surface finish will have a substantial impact on performance. Surface finishes can be applied mechanically (usually with abrasives) and chemically.
Understanding how chemical and mechanical treatments will affect the characteristics of the surface and will enable the best possible outcome for the client and the structure. Chemical treatment can be used to improve the corrosion performance of the steel, and hence its appearance in service.
Stainless steels resist corrosion best if they are clean and smooth. Clean means being free of contaminants on or in the surface that can either react with the steel (like carbon steel or salt) or that create crevices or other initiation points where corrosion can start.
Smooth means having a low surface area at the 'micro' level. Mechanically abrading the surface can roughen the steel's surface and may also embed unwanted particles.
The common feature of chemical treatments is that they all clean the surface of the steel. They may also smooth or roughen the steel surface, or leave it unaffected depending on which process is chosen. But if carried out properly, they all increase the corrosion resistance.
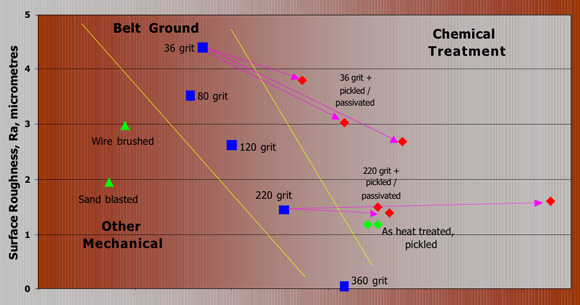
Corrosion resistance improves as you go to the right of this graph. The graph shows the relative importance of the smoothness of the surface and chemical treatment of the surface. They can be used together to get the best corrosion resistance. The study reported by G. Coates (Materials Performance - August 1990) looked at the effect of various methods of treating an artificial welding heat tint on grade 316, 2B surface.
Stainless Steel Products
During steel making, sulphur in the steel is controlled to very low levels. But even at these levels sulphide particles are left in the steel, and can become points of corrosion attack. This 'achilles heel' can be improved greatly by chemical surface treatment.
Most bar products will be slightly higher in sulphur when produced, so chemical treatment to remove inclusions in the surface of these products becomes more important.
Generally mill finishes for flat products (sheet, plate and strip) will be smoother as their thickness decreases.
A No 1 finish on a thick plate may have dimples or other imperfections and a surface roughness of 5 to 6 micrometres Ra.
A typical 2B cold rolled finish on 1.7mm thick sheet might have a surface roughness of 0.2 micrometres Ra or better as shown in Mill Forms.
New surfaces will be created during fabrication processes, (eg cutting, bending, welding and polishing). The corrosion performance of the new surfaces will generally be lower than the mill supplied product because the surface is rougher, or sulphide inclusions sitting just under the surface have been exposed or mild steel tooling contamination may have occurred.
Chemical treatments correctly performed can clean the surface and ensure the best possible corrosion performance.
Chemical surface treatments can be grouped into four categories:
- Pickling - acids that remove impurities (including high temperature scale from welding or heat treatment) and etch the steel surface. 'Pickling' means some of the stainless steel surface is removed.
- Passivation - oxidising acids or chemicals which remove impurities and enhance the chromium level on the surface.
- Chelating agents are chemicals that can remove surface contaminants.
- Electropolishing - electrochemical treatments that remove impurities and have the added beneficial effect of smoothing and brightening the surfaces.
Pickling
Mixtures of hydrofluoric (HF) and nitric acid are the most common and are generally the most effective. Acids are available as a bath, a gel or a paste.
Commercially available mixtures contain up to about 25% nitric acid and 8% hydrofluoric acid. These chemicals etch the stainless steel which can roughen and dull the surface.
Care is required with all these chemicals because of both occupational health and safety and environmental considerations. HF is a Schedule 7 poison which has implications for sale or use in most states. See ASSDA's Technical Bulletin on this subject.
Passivation
Nitric acid is most commonly used for this purpose. Passivation treatments are available as a bath, a gel or a paste. Available formulations contain up to about 50% nitric acid and may also contain other oxidisers such as sodium dichromate. Used correctly, a nitric acid treatment should not affect the appearance of the steel although mirror polished surfaces should be tested first.
Passivation works by dissolving any carbon steel contamination from the surface of the stainless steel, and by dissolving out sulphide inclusions breaking the surface.
Nitric acid may also enrich the proportion of chromium at the surface - some chelants are also claimed to do this.


Pickling and passivation (L-R): before and after treatment of fuel tanks for storing helicopter fuel on ships. Photos courtesy of Alloy Engineers and MME Surface Finishing.
Chelants
Chelants have chemical 'claws' designed to selectively clean the surface.
The carboxylic acid group COOH is the basis for many chelants which are used in cleaners, water softening and lubricants. The pH and temperature must be correct for the chelant to do its job. Turbulent rinsing of pipes and vessels afterwards is important.
Cleaning by chelating agents tends to be based on proprietary knowledge and systems, and is less standardised than the other methods described.
The successful use of these systems needs to be established on a case by case basis.
Electropolishing
Most commonly phosphoric and sulphuric acids are used in conjunction with a high current density to clean and smooth (by metal removal) the surface of the steel.
The process preferentially attacks peaks and rounds valleys on the surface and raises the proportion of chromium at the surface.
The technique can have substantial effect on the appearance increasing lustre and brightness while only changing the measured roughness by about 30%.

Electropolishing of the example on the right effectively removed contamination including heat tint and smoothed the surface lifting lustre and reflectivity compared with the untreated example on the left.
Precautions
For chemical processes that etch the stainless steel, reaction times will increase with increasing grade.
More care is required with 'free machining' grades and these will usually require substantially less aggressive chemicals. The sulphur addition in these steels makes them readily attacked by chemical treatments. Care is also required when treating martensitic or low chromium ferritic stainless steels.
Detailed recommendations for each grade of stainless steel are given below.
The four categories of treatment are detailed in a number of Standards, but the most commonly used are:
- ASTM A380 Cleaning, Descaling and Passivation of Stainless Steel Parts, Equipment and Systems.
- ASTM A967 Chemical Passivation Treatments for Stainless Steel Parts.
- ASTM B912 Passivation of Stainless Steels using Electropolishing.
These very useful documents give detailed recommendations on many aspects of selection, application and evaluation of these treatments. Highly recommended reading.
Dirt and grease will mask the surface from treatments outlined above. Therefore, the steel surfaces must be free of these agents before applying chemical treatments.
Many of the chemical treatments described contain strong acids. Before disposal they will require neutralisation. Check with your local authority concerning the requirements for trade waste, neutralisation and disposal.
Many of the chemicals described above will be classified as hazardous substances under State OHS legislation, with implications for purchasing, transport, storage and handling.
Chemical treatments are useful tools in cost effectively achieving peak performance with stainless steels. With appropriate training, hazards associated with their use can be managed.