Raw material price fluctuations and increasing demand for stainless steels have driven demand for lower cost alloys as alternatives to the traditional “300” series steels. This has been met through a range of existing and new, innovative steels with different properties, performance and availability. But as with the traditional stainless steels you can't tell what they are by looking at them. This article describes a range of test methods available for grade confirmation. The method used depends on the budget, size of job and the potential consequences of having the wrong alloy. Some of these tests are quantitative, giving actual percentages of each element, and others are qualitative tests showing just the presence of absence of an element or property. Some tests are very portable so are ideal for on-site testing, but others require fully-equipped laboratories. The martensitic grades (high hardness, magnetic grades used for making knives and blades) are not considered in detail in this technical note.
Download ASSDA Technical FAQ 4 (PDF)
WHY TEST?
Contract documents may require formal test certificates. These are issued by the mill and unless there is reason to doubt them, this is sufficient. However, sometimes a positive material identification (PMI) is required for safety critical items such as LPG valves - this is an individual confirmatory analysis on each finished item.
Some products may be lacking in documentation and traceability; the most common concern is stock mixed in storage or as incoming scrap.
Unexpected poor performance often prompts calls for material testing. Such testing removes one variable in things that might have gone wrong but the cause is more frequently inadequate surface finish or errors in design or fabrication.
Finally, reverse engineering of an existing product often requires detailed materials information - generally more than just composition.
WHAT LEVEL OF TESTING IS REQUIRED?
Simple tests could cover differentiation between carbon and stainless steel, or between 304 and 316, or between 300 series and 200 series, or ferritic/duplex and austenitic grades.
Full laboratory chemical analysis is needed for some cases (such as differentiating between low and standard carbon grades) or when it has become a legal rather than a technical issue.
Full mechanical and metallurgical analyses may also be required if strength or hardness are essential design elements. If the material has undergone cold working or subsequent surface modification such as plasma vapour deposition (pvd) or nitriding, then the required investigation could be extensive - and expensive. The summary table shows results for three tests that can be used to distinguish between grades.
TABLE: Summary of rapid test results for distinguishing between grades
| Test | 200 series austenitic | 300 series austenitic | 400 ferritic | Duplex |
| Magnet | Not attracted* | Not attracted* | Attracted | Attracted |
| Mo Spot | Some proprietary grades positive | 316, 317, 904L & 6% Mo grades positive | Not 409 or 430 but 444 and higher grades positive | 2205, super duplex positive |
| Mn Spot | Positive by definition | No colour | No colour | Lean duplex positive |
NOTE: *Cold worked austenitic grades may be slightly magnetic with a greater effect if the deformation is severe.
SIMPLE PHYSICAL TESTS
Appearance is not a reliable indicator of the grade of stainless steel as the differences are determined more by surface treatments than alloy composition. There are only slight differences in density between stainless steel grades (7700-8000 kg/m3) and density determination is not a convenient method. It is rarely used as a sorting tool.
Magnetic response 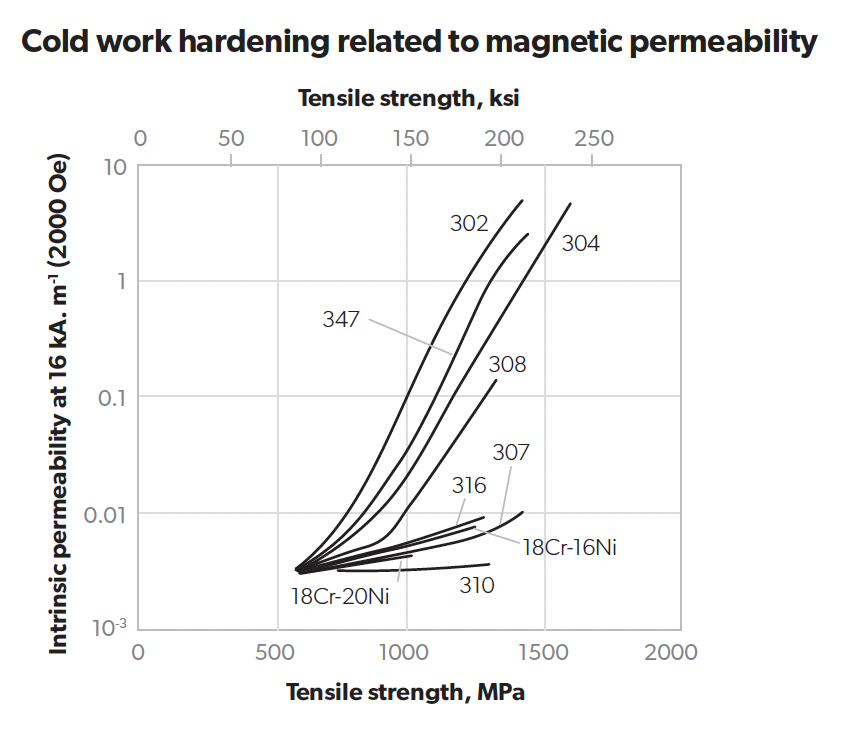
A widely accepted but sometimes misunderstood test is with a magnet. Duplex, super duplex, martensitic and ferritic stainless steels are strongly attracted to a magnet, while annealed austenitic stainless steels are not. However, cold worked austenitic stainless steels can develop a magnetic response, so cold formed ends to a vessel, cold formed bolts and particularly cold drawn wire or bar may be affected by a magnet. This applies to both the conventional CrNi 300 grades and CrMn 200 austenitic grades. The strength of the magnetic effect is related to the relative permeability. The graph below shows the different effects of the same level of cold work (e.g. by drawing) on various austenitic grades. For austenitic grades, more deformation or cold work results in higher strength. The austenitic grades with higher nickel or other austenitising elements (310 or 316) show a much lower magnetic response. Mild steel has a relative permeability between 200 and 2000. The relative permeability of duplex and ferritic stainless steels is in the hundreds.
PORTABLE CHEMICAL TESTS
The simplest chemical test to distinguish carbon steel from a magnetic stainless steel is to apply a drop of nitric acid. The carbon steel will react - the stainless will not.
There are proprietary kits designed to test for a specific element. These are simple chemical tests that use an acid to dissolve a small amount of the stainless steel. Alternatively, a small battery can be used instead of an acid. In both cases, the dissolved metal reacts with other chemicals to give a colour. The battery test is much quicker and its rate is not dependent on temperature. All of these spot tests will mark the surface, but the amount varies between tests - check in an inconspicuous location if this is important.
Although these qualitative tests are convenient and quick, if you only require a couple of tests a year, then it may be cheaper and more thorough to run a full laboratory test.
Molybdenum 
The most common test uses a single drop of solution to distinguish between low and high molybdenum content. The "Moly Drop" test will distinguish between 304/304L and 316/316L, but the test will also give a positive result with 317/317L, 904L, the 6% Mo grades, 444, 2205 and the super duplex grades. The test requires a clean, dry, grease-free surface and it sometimes helps to light abrade the surface.
The yellow or clear drop will darken after a few minutes but the reaction speed is slower if the surface is cold. It is a comparative test. The test is more reliable if a known sample of the required grade is tested with the unknown. If the test sample is to be used in service, then the chemicals should be washed away immediately after the test.
An electrochemical test uses less aggressive chemicals and an electric current from a battery. In this case, a tell-tale pink colouration on a filter paper shows molybdenum is present.
Manganese
The increasing use of high manganese (200 series) stainless steel has led to several manganese test kits operating on the same principal as the electrochemical test for molybdenum.
The semi-quantitative results of a kit test for manganese are shown in the photograph.
Apart from the recent low nickel, high manganese stainless grades, there have been specialist 200 series grades used in generators, as higher strength marine shafting grades and for anti-galling applications.
Sulphur
A practical and rapid comparative test for a high sulphur (free machining) stainless steel (303 and 430F are the most common) is to prepare sulphur prints using photographic paper soaked in 3% sulphuric acid for several minutes. The treated paper is pressed onto a cleaned surface for about five seconds. High sulphur levels are shown by a brown colour. If the test item is to be put in service, the acid residue must be removed immediately. Sulphur-containing free machining steels have lower corrosion resistance, unlike the calcium-treated improved machinability grades.
PORTABLE INSTRUMENTAL TECHNIQUES
There are two basic techniques. These automated instruments are expensive and would normally be used for large projects, or by scrap metal merchants, manufacturers or specialist NDT contractors.
Spark spectroscopy
Spark spectroscopy requires a flat surface preferably about 20 mm in diameter. An electrical spark is generated and the elemental concentration is measured by the intensity of the specific colours. In automated instruments, the spectrum is compared to a library of data and percentage composition is calculated for each element. A sparking mark is left on the surface. The instrument's accuracy tends to be lower than a laboratory instrument and exposure to air excludes measuring nitrogen.
The older "Metascopes" were also spark spectroscopes but relied on visual comparisons of line brightness so their accuracy was very operator-dependent.
X-ray fluorescence
The second broad method is x-ray fluorescence (XRF). Older instruments use one or more radioactive sources although more recent miniaturisation of x-ray tubes means that some instruments generate x-rays directly. Provided that the surface is clean and smooth and the measurement is for long enough to give good statistics (typically between 20 and 60 seconds), then the alloy can be identified. However, it cannot analyse for light elements, especially carbon or nitrogen. The units are light and easy to use as shown in the photograph.
One advantage is that results can be directly downloaded into a computer. The XRF testers leave no residual mark on the steel surface.
LABORATORY MEASUREMENTS
Larger and more accurate versions of the spectroscopes and XRF instruments are used by chemical analysis laboratories. The steel makers use these instruments to generate the data for their inspection certificates and are also available for public testing through independent testing companies who can be found via the National Association of Testing Authorities, Australia (NATA): www.nata.com.au.
Atomic Absorption (AA) or Inductively Coupled Plasma Spectroscopy (ICP) techniques use laboratory instruments after a sample has been digested in (usually) a mixture of acids. This is slow and may be more expensive than a spark test but it will give a more complete and reliable result. Carbon requires a separate (LECO ignition) test.
IN SUMMARY
Probable testing can give rapid and onsite but sometimes qualitative results - these are very useful for sorting grade mixes. Some grades cannot be sorted by purely qualitative tests, but may require either portable instrumental analysis or full laboratory testing. In some cases, other tests such as hardness or metallographic examination may be needed to fully understand the metal.
WHICH TEST?
-
Is it 430/2205 or 304/316? A magnet will be strongly attracted to 430 and 2205, but only weakly to deformed parts of 304 or 316.
-
Is it 430 or 2205? Both are strongly magnetic but only duplex 2205 will give a positive moly drop test result.
-
Is it 304 or 316? A moly drop test will give a positive result with 316.
-
Is it a low carbon grade? Only a spark spectrometer can distinguish between low and standard grades.