
One of the most common misunderstandings in specifications for stainless steel fabrication relates to the post-fabrication treatments to restore or enhance the corrosion resistance.
The surface treatment processes invoked vary between pickle and passivate, passivate, or sometimes simply pickle. Needless to say, whilst pickling and passivation are two distinct processes, a lack of clarity can cause some confusion between the owner and the builder/fabricator about what is expected and required.
This article briefly outlines the factors that affect the corrosion resistance of stainless steels, what surface treatments can be used and how they affect the steel’s surface to improve corrosion resistance.
Corrosion Resistance and its Controls
Stainless steel is resistant to aggressive environments because of a very thin, self-repairing, chromium-rich complex, oxide film present on the surface of the steel. It is not completely impervious, but it dissolves many orders of magnitude more slowly than it reforms. The passive layer is more resistant for alloys with more chromium, molybdenum and nitrogen. This is the reason for the empirical, composition based Pitting Resistant Equivalent (PRE(N)) index which is often used as a ranking tool in selecting which stainless steel will be used in new applications. However, the alloy composition is not the only control of the passive film’s strength, and hence its corrosion resistance. There must also be an adequate supply of oxygen and moisture to maintain the integrity of the passive film. This requires either good design or a maintenance program – and preferably both.
For a specific alloy, i.e. a specific PRE(N), the passive film (and hence the corrosion resistance) can be improved by chemically oxidising the steel’s surface. Air and water are good and the ASTM standard dealing with passivation (A967 Standard Practice for Chemical Passivation Treatments for Stainless Steel Parts) advises that for many environments, no further treatment is required for satisfactory service. However, oxidising or chelating chemical treatments will provide better corrosion resistance.
Roughness
Corrosion resistance is indirectly improved if the surface is smooth and clean (free of contaminants) to facilitate the self-renewal of the passive film. For abraded surfaces there is also a critical surface roughness of 0.5μm Ra that should not be exceeded. This is recognised in surface finish 2K in EN 10088.2. It seems that for steels, the size of abrasives causing this roughness is too large to cut the surface cleanly and leaves rough edges and metal debris which can accumulate dirt and corrosives – hence more rapid corrosion with coarser polishing.
Contamination
The bête noir of stainless steel: carbon steel contamination. If it is not removed, the stainless steel will rust. In marine environments, it will collect chlorides and cause large rust stains and small pits in the stainless steel. If it is mechanically removed, it is likely that the smeared steel will leave a larger rust stain, although it may be less intense. Acid treatments can remove carbon steel deposits and have the added advantage that they can also remove surface breaking manganese sulphide (MnS) inclusions. These MnS inclusions do not have a passive film and act as initiating points for corrosion.
Welding
If you have welded your fabrication and there are rainbow coloured bands along the welds, they are zones where the passive film has been destroyed. Under the darker colours, there will be a wedge-shaped layer with a lower chromium content than the bulk stainless steel. Corrosion will initiate in these coloured bands. The weld tint colours can be mechanically removed provided the grinding is not too rough. Chemical removal by pickling is often a better option.
Pickling
Pickling uses a mixture of nitric and hydrofluoric (HF) acids. The wide range of concentrations and exposure times are described in ASTM A380 Standard Practice for Cleaning, Descaling and Passivation of Stainless Steel Parts, Equipment and Systems. Typically the nitric concentration is up to 10 times the HF concentration, but pickling is slow unless the HF is more than about 3%. Longer pickling times are required at lower temperatures or if a high alloy is being used, i.e. super duplex takes longer than duplex which requires longer than 304. If a paste is used, the contact area acid gets exhausted unless it is stirred, e.g. with the application brush. Thorough washing is needed to remove all residue even from crevices and, to avoid stains, it is important not to allow acid or rinse water to dry on the surfaces.
OHS and environmental considerations mean that using a pickling contractor is easier, safer and ensures the appropriate disposal of acid and pickled heavy metals. Contractors will often use a temperature-controlled, stirred tank or, sometimes, a spray pickling solution in an acid-proof and bunded bay. Unless an additional level of passivation is required for a very aggressive environment, the outcome is a pickled item that is passive.
Chemical Passivation
The traditional and very effective acid is nitric, typically between 15% and 25% for about two hours, although it is not uncommon to drop machined parts into a bucket of nitric acid for half a shift. The passive film is significantly strengthened and the ratio of chromium to iron in the surface layers can exceed 1 – compared to <0.4 in the bulk. Nitric acid will also remove rust stains and sulphide inclusions plus, more slowly, carbon steel smears. Phosphoric acid will remove rust and sulphide inclusions, but it is not oxidising and will not strengthen the passive film. Another method of strengthening the passive film of a chemically-clean surface is to use a hydrogen peroxide solution – lots of free oxygen and only water residue.
There are other acids that will strengthen the passive film and dissolve carbon steel and inclusions, but by a different method. Citric and oxalic acids and EDTA all have a carboxylic acid [O-C-OH] atomic structure, and once the acid dissolves the unwanted metal, the positive ion is trapped by the negative oxygen atoms in a process called chelating or sequestering. This process is used in wastewater treatment to remove metals. In passivation, it is important to rinse thoroughly. Chelating treatments are widely used in the food industry as formulations which include biocides, so the citric acid does not contribute as a food source.
There are a number of special cases detailed in ASTM A380 which require care when pickling:
• Sensitised or hardened (nitrided or carburised) areas may suffer intergranular attack.
• Free machining stainless steels requires an inhibitor or it will pit.
• Martensitic stainless steels can suffer hydrogen embrittlement.
All of the above methods are chemical treatments which are quite traditional and generally well applied. Further information is provided in ASTM A380, which also details test and inspection methods to confirm surface cleanliness.
Three Definitions
CLEANING Removal of contaminants such as soil, grease, oil, etc. using low-chloride detergents and/or solvents to allow free access for water and oxygen to grow the passive film.
The bulk material is not affected and the surface looks brighter. Chlorinated solvents may be a risk as residues can degrade if heated and may cause pitting. In vessels or pipework, it is important to drain and dry the surfaces.
PICKLING The removal of any high temperature scale and any adjacent low chromium layer of metal from the surface of stainless steel by chemical means.
It also removes embedded or smeared carbon steel, inclusions and loose flakes of stainless steel left from abrasives.
It will leave a matt finish, which may be paler if the pickling is extended. It provides a passive surface immediately on rinsing – hence you pickle and get a passive surface.
PASSIVATION The treatment of the surface of stainless steel, often with acid solutions (or gels), to remove contaminants and promote the formation of the passive film on a surface that was freshly created, e.g. through grinding, machining or mechanical damage. It will remove acid soluble inclusions such as MnS.
Clean humid air will form a passive film on clean stainless steel and the appearance will not change.
Chemical passivation strengthens the passive film and typically takes an hour or so at ambient temperatures. Air passivation is adequate unless the environment is very aggressive for the grade.
|
1. Rusting steel contamination from shearing stainless sheet. Photo courtesy of Graham Sussex. |
2. Rainbow oxide from poor gas shielding during welding. Photo courtesy of HERA. |
 |
 |
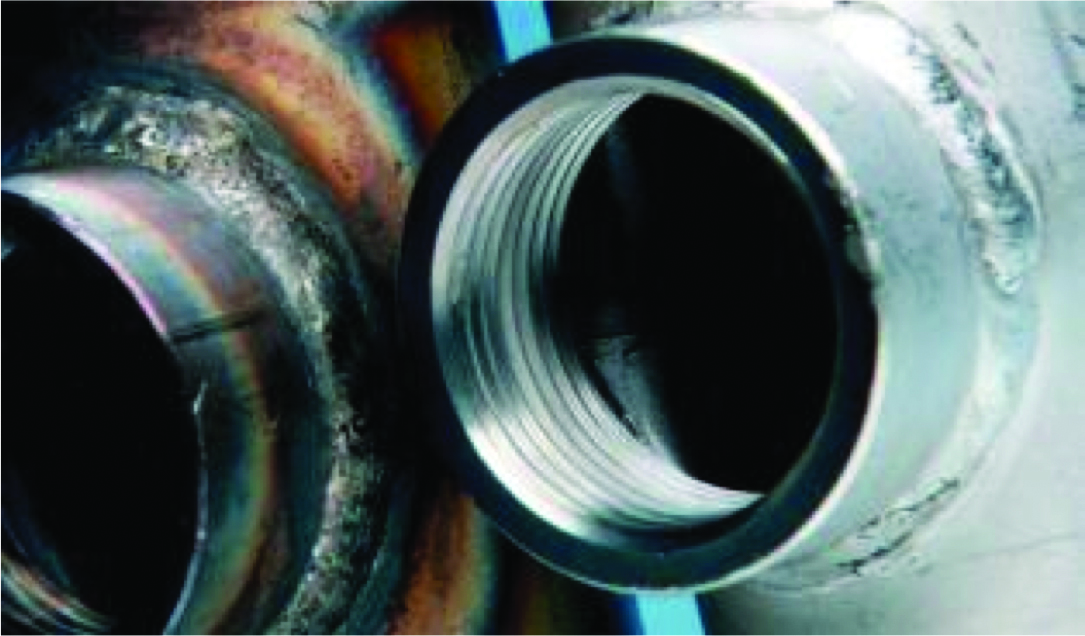
| 3.Before (left) and after (right) pickling of welded fitting. Photo courtesy of Graham Sussex. | 4. Welded components after pickling to remove heat tint and possible steel contamination. Photo courtesy of Australian Pickling & Passivation Service. |
 |
 |
This article featured in Australian Stainless magazine - Issue 64, Summer 2018/19.