
Shielding gases form an integral part of all conventional welding processes.
They serve multiple functions but are primarily there to shield the weld pool from the atmosphere and to provide a medium which can allow the flow of electricity from an electrode to a workpiece. Even processes that do not have an external gas supply such as Manual Metal Arc Welding (MMAW or MMA or SMAW) and Gasless Flux-cored Arc Welding (FCAW) all have a shielding gas which is generated by the decomposition of the flux in the presence of the welding arc.
The shielding gas can also have an effect on arc stability, weld shape and depth of penetration as well as the mechanical properties and metallurgy of stainless steel weldments.
The gas shielded processes such as Gas Tungsten Arc Welding (GTAW or TIG) and Gas Metal Arc Welding (GMAW or MIG) use shielding gases of a variety of compositions depending on the application. As the electrode in GTAW is made of tungsten, the shielding gas is typically argon or helium to prevent oxidation of the electrode. This restriction does not apply to GMAW and therefore the gas composition may include active gases such as carbon dioxide and oxygen. Small quantities of other gases such as nitrogen and hydrogen can be utilised with both of these processes as they are particularly advantageous for the welding of stainless steel. While neither gas is inert by definition, they can be used with GTAW as neither react with tungsten.
There are three key properties of the shielding gas which control the way the weld pool behaves; the ionisation potential (how easily an atom will give up an electron), the thermal conductivity of the gas, and finally the surface tension between the weld pool and the shielding gas.
Ionisation potential
The shielding gas allows transfer of electrons between the electrode and the workpiece. Upon arc initiation, electrons are emitted from either the workpiece or the electrode depending on which is positively charged. These electrons collide with gaseous atoms which results in these atoms liberating one of their electrons which results in a chain reaction that sustains the arc. The ionisation potential of the gas is the ease with which they will give up an electron. ‘Hotter’ gases are those which require more energy to ionise or release an electron. Helium has a higher ionisation potential than argon, so has a higher arc voltage and hence a higher heat input for the same current and arc length.
A similar principle applies to molecular gases (H2, N2, O2, CO2) which dissociate in the arc into individual atoms and then recombine upon cooling, releasing energy in the process. Argon is often mixed with small amounts of other gases to improve weld penetration.
Thermal conductivity
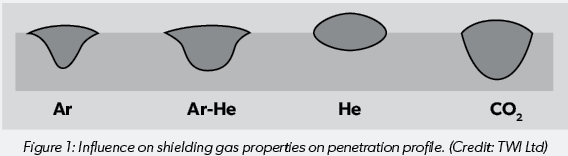
The thermal conductivity of a shielding gas affects its ability to transfer heat across the arc. It influences the radial heat loss from the centre to the periphery of the arc column as well as heat transfer from the arc to the molten weld pool. Gases with low thermal conductivity such as argon will tend to have a narrow hot core in the centre of the arc and a considerably cooler outer zone. The result is a weld with a narrow ‘finger’ at the root of the weld and a wider top. On the other hand, helium has a high thermal conductivity, so heat is more evenly distributed across the arc, but as a result the depth of penetration is lower. Mixing gases allows combination of the advantageous properties of each gas while limiting the drawbacks.
Surface tension
Surface tension affects the bead profile of a weld. Picture how water beads up on a newly polished car. This is undesirable in welding as it creates a steep angle between the weld and the parent which could lead to defects such as undercut, lack of sidewall fusion and decreased fatigue performance. This is another reason why pure argon is not used as a shielding gas for the GMAW process.
Gas components
Oxygen
Though seemingly counterintuitive as it is well known that hot metals oxidise, small amounts of oxygen are often added to shielding gasses for the GMAW process. Small amounts of oxygen reduce the surface tension between the molten weld pool and the surrounding atmosphere. Lower surface energy results in a flatter and smoother weld bead with less tendency to undercut the parent metal. To minimise alloy losses by oxidation, oxygen content is typically limited to 2%. The heat tint will be more severe than for a weld without oxygen additions to the shielding gas.
Carbon Dioxide
GMAW also utilises CO2 as a constituent of the shielding gas. A common concern with stainless steels is embrittlement and corrosion through sensitisation due to chromium carbide formation, but the carbon pickup from CO2 has been demonstrated to be low enough that the resultant weld metal still achieves the required (≤0.03%) carbon content for L grade designation. The chosen CO2 content is therefore more about penetration and wetting than it is about carbon pick-up. Carbon dioxide contents in GMAW are typically 2-5% while flux-cored wires utilise 20% mixtures with argon or even 100% CO2.
Hydrogen
Unique to austenitic stainless steels is its immunity to hydrogen cracking – except possibly in very heavily cold-worked material. This allows the addition of hydrogen to the shielding gas in quantities from 2–15% providing more heat in the arc and better penetration. Hydrogen quantity for manual welding is usually restricted to 5%, with the higher concentrations limited to automated process such as orbital GTAW. Hydrogen cannot be used as a component of the shielding gas for ferritic, martensitic or duplex stainless steels due to a risk of cracking.
Nitrogen
Nitrogen is a useful shielding gas additive for duplex stainless steels which contain dissolved nitrogen. It is added to increase pitting resistance and in acting as an austenite stabiliser to create a balanced ‘duplex’ microstructure in the weld, especially for thin materials which cool too rapidly to allow sufficient austenite to form. Nitrogen can be added to both the welding gas and the purge gas to prevent the loss of nitrogen during welding.
This article has dealt with gases for the active side of a weld. When welding tube or pipe, it is normal to feed an inert gas such as argon or nitrogen into the tube or pipe to maintain low oxygen levels and minimise heat tint formation to no more than pale straw. This usually requires a sensitive oxygen meter or possibly previously proven purging practices. In thick sections, purging must continue for all passes. Nitrogen purging of duplex root passes will improve the corrosion resistance but may also upset the phase balance. Hydrogen additions have been used in purge gases for both austenitic and duplex welds to minimise heat tints.
the tube or pipe to maintain low oxygen levels and minimise heat tint formation to no more than pale straw. This usually requires a sensitive oxygen meter or possibly previously proven purging practices. In thick sections, purging must continue for all passes. Nitrogen purging of duplex root passes will improve the corrosion resistance but may also upset the phase balance. Hydrogen additions have been used in purge gases for both austenitic and duplex welds to minimise heat tints.
