
Posted 31 July 1993
Quality is the buzz word of the last part of the 20th Century and manufacturers ignore quality control at their peril. With proper attention to detail, stainless steel will provide satisfactory service for many years. The Chrysler building in New York has a stainless steel finish that is in excellent condition after 60 years.
Stainless steel is a quality material and for products made from it to meet the demanding quality standards required, there are some special features that those used to fabricating other materials must watch if their product is to meet their customer's expectations.
Surface contamination is one of the danger areas.
Contamination with carbon steel - embedded iron.
If stainless steel is fabricated with tools previously used for forming mild steel there is almost certain to be carbon steel pick up from these tools onto the stainless steel surface. Grinding wheels used for both carbon steel and stainless products or machining stainless steel with conventional steel cutting tools are particular dangers.
These iron particles will form a galvanic couple with the stainless steel and quickly rust. At this early stage they could be cleaned away with a mild abrasive with little or no damage to the stainless steel surface. If they are left, it could be another story!
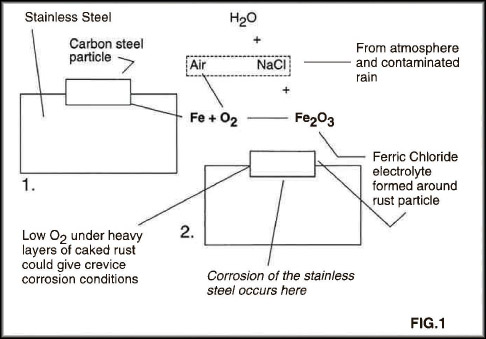
When the carbon steel particle rusts, as it is sure to do, it will form iron oxide that will eventually turn into ferric chloride, Figure 1. There are always chloride ions available particularly in marine atmospheres. The resultant ferric chloride will rapidly attack the stainless steel and pitting corrosion will result, generally visible as rust in a pattern related to the source of contamination.
In the fabrication area mark up tables, and the platens of cutting and bending equipment should be covered with cardboard or plastic that is frequently cleaned down or replaced. Crane hooks and forklifts should be similarly protected. A protective plastic film on the surface can limit the amount of pick up that can occur during handling.
Embedded iron will not only arise from fabricating equipment. Hot rolled sheet or bar will not be treated for surface contamination when delivered. Ground finished products are safer. During the manufacture of large vessels carbon steel particles or swarf from other areas of the shop can also be transferred into the tank on workers' boots and clothing and care must be taken to avoid this.
Small particles of embedded iron can be removed by nitric acid applied either as a paste or immersion. Heavier contamination may require a nitric/hydrofluoric acid treatment. These processes are know passivation or pickling.
Other Contamination
There are other dangers during fabricating that can generate crevice corrosion conditions. Adhesive tape is the classic case. The area under the tape is low in oxygen and if moisture can seep under the tape - and it most probably can - a crevice is created where corrosion can occur.
The same sort of crevice can be formed under grease, crayon markings or residual adhesive left after the protective film is removed. Also, some films, if left exposed to the sun's UV radiation for more than a few hours harden and both the film and underlying adhesive are very difficult to remove.
In most industrial areas deposits can build up on the surface. This problem can be eliminated by washing the surface down - a good rule of thumb for architectural applications is to wash exterior stainless steel at about the same frequency as the building windows require washing. Maintenance manuals supplied with stainless steel fixtures or components should specify this cleaning requirement.
Welding also requires attention. Weld spotter and potential crevice corrosion points at the initiation and run out points of a weld run can be a problem as can contamination with grease, crayon marks or carbon steel in the weld area, including an edges, is essential.
Another area of concern is the oxide film formed during welding and the concurrent decrease in alloy content of the material under the weld. In all cases it is good practice to passivate the steel after the welding.
Written by Noel F. Herbst, Director, Nickel Development Institute of Australasia
This article featured in Australian Stainless Magazine - Issue 1, July 1993.